About Us
Corporate Profile
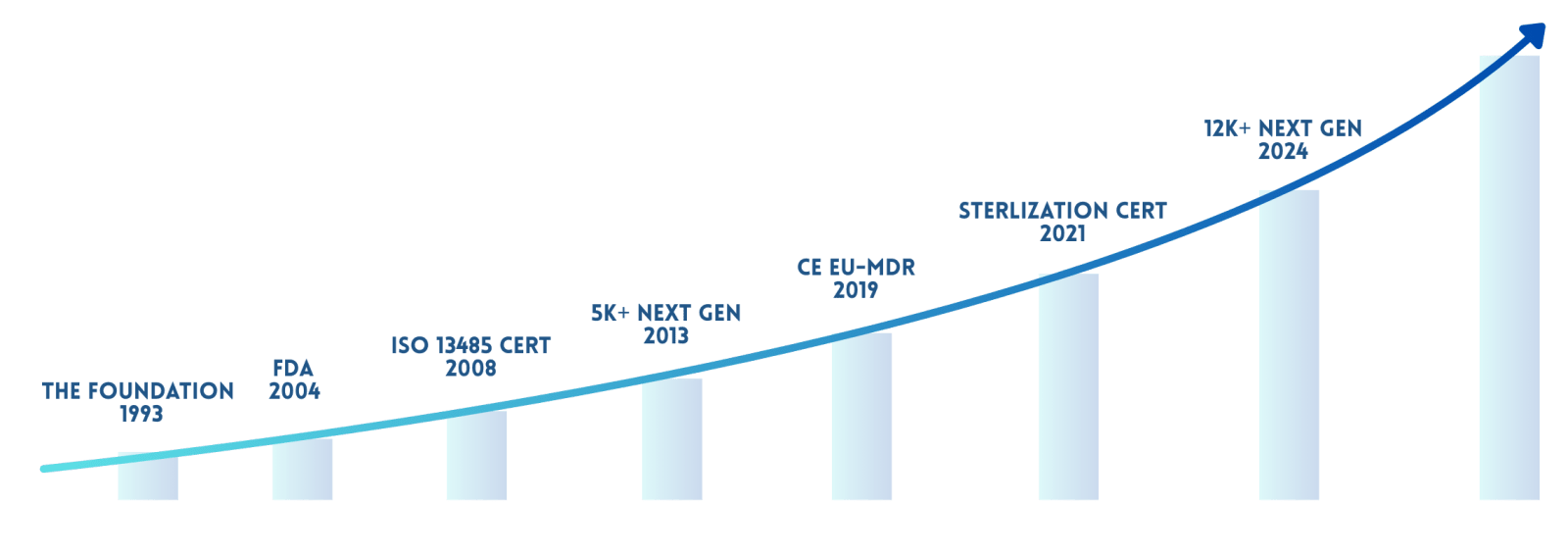
In October 2005 The Central Govt. of India issued a notification S. O. No.1468(E) dtd. 6th Oct – 2005 wide which The Orthopaedic Implants had been covered under Drugs & Cosmetics Act 1940. These notified devices are to be manufactured by only valid Medical Devices Mfg. License holders approved by CDSCO, India. We took very early steps to fulfil the regulatory requirements by setting up all new state of the art manufacturing unit at Vadodara in 2007.
The company successfully complied with the standards laid down by The Indian FDA and got the manufacturing Drugs License in form-28 on 26th June, 2009 jumping among early top five companies of India and later complying with New MDR 2017 MD-9 License for Sterile and Non Sterile Implants. Our manufacturing unit is equipped with all modern manufacturing facilities. The manufacturing activities are carried out under ISO 13485:2016 standards and strict supervision of technically competent persons and management. Our vision is to offer quality products at affordable rates with unbeaten service back up.
Ortho Max® is located at most developed engineering industrial estate in Vadodara,one of the fastest growing city and engineering hub of Gujarat State, The city is well known for its rich culture and royal heritage of King Sir Sayaji Rao Gaekwad 3rd. The engineering industries of this city stand best in the country with all hi-tech facilities and advanced resources. Companies like Reliance, Bombardier, FAG, L & T, Siemens, ABB, SUN Pharma, Alembic etc. have big set up in this city.
Ortho Max® History
Ortho Max® Legacy Forged in Vision, Strengthened by Generations.
For over 33 years, under the active and visionary leadership of MD. Mr. Suresh Premji Dedakiya, Ortho Max® has been a pioneer in orthopaedic and maxillofacial implants earning a reputation of trust, quality, and innovation in the healthcare industry.
Today, this legacy is being strengthened by the next generation Mr. Prashant Dedakiya and Mr. Yash Dedakiya who, for the last 10+ years, have been working closely under his mentorship, bringing in hands-on expertise, cutting-edge German and Japanese technologies, and a modern global outlook to the company’s journey forward.
Together, they represent a perfect blend of wisdom and energy, tradition and transformation, leading Ortho Max® toward a new era of excellence.
Ortho Max® stands at the intersection of experience and innovation continuously redefining what modern healthcare truly means.
Management Message
In today’s modern technology era, surgical treatments have become easy, accessible and innovative but expensive. It should be the responsibility of every medical company to make up with the pace of changing trends of modern medical science by keeping the cost in control making surgical treatment affordable to all looking at the demography of Indian subcontinent. Globally the challenges are big, execution is difficult, but we have to come up to the expectations of our user surgeons and patients.
We at Ortho Max® strive hard to meet the expectations of our users by offering international standard quality product which is easy accessible and affordable without compromising with the most important word of medical devices “Safety”.
It is our dedication-hard work-passion and business ethics which makes us different from others with unmatched quality, personalized services and ethical business practice across Industry. We reiterate our commitment towards the medical fraternity to provide innovative surgical trauma solutions accessible and affordable to all on time – every time.

Managing Director
Mr. Suresh Dedakiya

Director
Mr. Prashant Dedakiya

Director
Mr. Yash Dedakiya

Quality Policy
Ortho Max® Team
The team behind the success of the company has a healthy presence of more than 100 members whose dedication and commitment have laid down a strong foundation of this company. Each member of this team is full of energy, enthusiasm and sincerity and is confident to give his or her best at all. The top management provides all the motivations and resources to achieve the desired objectives.
We deserve the satisfaction of hundreds of our valued customers, distributors/ dealers and employees without whom we would not have come to the destination we have reached today…


Research & Development
All the research & development activities are conducted indigenously by a team of experienced and competent members. The new products are developed under the guidance of senior surgeons & technical consultants after proper validations and trials. A true to model accuracy is designed and achieved with highest precision & verification by a team work of mechanical engineers & technicians.
Infrastructure
The manufacturing unit is set up in a sprawling area to produce high quality medical devices meeting all international standards. The layout of premises is perfectly designed to comply with the regulatory requirements and achieve optimum output under efficient working environment. Each department is well equipped with all modern facilities. All critical machine operations are performed on Japanese/Swiss technology Computerized Numerical Control (CNC) Machines giving highest quality & uniformity. The back process of implants is performed on German Technology Polish Plant to achieve edge free surface and best aesthetics. The Unit has got in house Micro Lab of ISO class 5 for Testing Sterility of Gamma Radiated Products. The final inspection- Laser marking and packaging activities are performed in class 8 clean rooms for complete hygiene and safety.
The unit has got best working environment for all the employees with provision of all safety & necessities. All the operations are carried out under one roof for better traceability and control, right from raw-materials landing to the finish product’s dispatch.

Ortho Max® Network
The sales network of Ortho Max® has touched almost all the corners of India and neighboring countries having one of the largest customer network in Orthopaedics- Oral/ Maxillofacial & Plastic Surgery.
Authorised Distributors & Dealers are appointed in almost all the Tier I-II & III level cities for round the clock services. We are a regular participant of all the annual conferences, seminars, workshops and trade exhibitions for almost 30 years.
Customers can send whatsapp message to +919998108750 to know about the nearest Distributor/Dealer. Ortho Max also provides direct customer sales & services wherever authorized dealers are not appointed.
We solicit new Distributor/Dealer’s enquiries in open territories and offer a very lucrative business opportunities with minimum investment and maximum ROI.
We appreciate and welcome your valuable suggestions for continual improvement.
Kindly post your feedback at info@orthomaxindia.net, admin@orthomaxindia.net
Call : +91 9998108750
Feedbacks
Clinical Voices: Why Experts Choose Our Implants









Ortho Max® plates, screws, drill bits , chisels, osteotomes, and some retractors for 14yrs. Implants and instruments are of good quality, user friendly, and affordable.